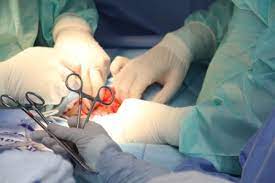
You may be eligible to submit an Ethicon surgical staples lawsuit for that defective product. In October 2019, the You.S. Meals and Medicine Management (FDA) initiated a category 1 recall—its most serious sort of recall—of Ethicon operative staplers. In accordance with the government organization, the product breaks down to correctly type staples that may increase the risk of medical difficulties.
There have reportedly been numerous accidents brought on by Ethicon medical stapler problems and also one death. Call an Ethicon Recall Legal professional to see in the event you match the standards for a Ethicon lawsuit staples.
Ethicon Medical Stapler Recall
There have been several recalls of surgery staplers through the years, showing just how high-risk they may be to make use of. In May 2013, Ethicon, a subdivision of medication huge Johnson & Johnson, given a Class-II recall for medical staples sold underneath the title Echelon. Echelon staples were utilised in many various kinds of surgical procedure, which includes belly stapling surgical procedure. The remember states that Echelon staples found in abdomen stapling ended up being reported to misfire and bust, jeopardizing severe problems.
On Oct. 30, 2019, the Federal drug administration introduced the category 1 remember of Ethicon’s Echelon Flex Endopath staplers. The FDA remember, established by the Johnson & Johnson subsidiary earlier in October, involves a variety of products, such as the subsequent:
ECHELON Flex 60 Endopath Stapler, Articulating Endoscopic Linear Cutter (EC60A)
ECHELON Flex 60 Operated Plus Lightweight Articulating Endoscopic Linear Cutter (PCEE60A)
ECHELON Flex 60 Run Plus Articulating Endoscopic Linear Cutter, 44cm Shaft Duration (PLEE60A)
ECHELON Flex 60 Powered Plus Articulating Endoscopic Linear Cutter, 34cm Shaft Span (PSEE60A)
These individual-user staplers were actually created to use on internal cells throughout minimally intrusive gynecologic, urologic, thoracic, pediatric, and standard surgical treatments.
In line with the FDA, these units might have a component that has run out of specification—leading to malformed staples. Malformed staples might lead to serious operative complications.Two patients were reportedly wounded by the standard devices each time a misfire result in their rectums being cut. Since Oct. 3, Ethicon has reportedly acquired seven reports of significant injuries then one report of loss of life.